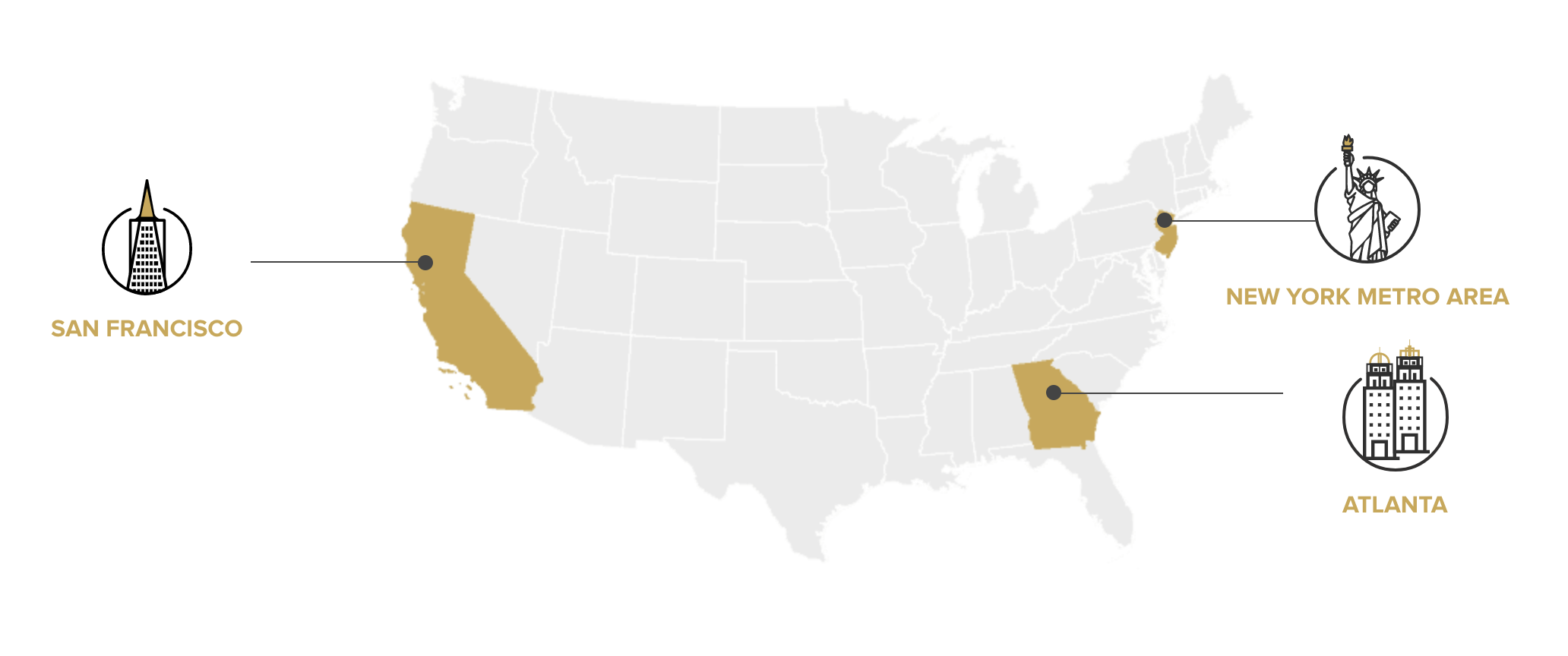
Based in the innovation hub of the Bay Area...
Caravel Group offers specialty consulting and marketing services
to help our clients achieve superior results.
We partner with industry, academia, and venture capital
to provide premier services to the life sciences industry.
Why Caravel Group
As a strategic partner, we provide medical communications, novel marketing services and meetings management support, leading our clients to achieve superior results. Caravel Group is a specialty consulting, medical communications, and events management company.
EXPERIENCE
INNOVATION
EXCELLENCE
EXPERIENCE
INNOVATION
EXCELLENCE








•
Backlit and wall displays
The client was also seeking to increase interest and drive enrollment in their clinical trials.
•
A client’s inflammation medical affairs team wanted to introduce their business unit as an emerging leader with a robust inflammation pipeline at annual global IBD and RA conferences.
•
Caravel developed scientifically compelling IBD and RA branded materials for the conferences, including:
•
•
•
•
•
Brochures
Booth wall banner
Digital slide show presentations
Sponsorship items: lanyards, water bottles and cup sleeves

•
A global medical affairs client was stretched thin: their internal and field-based medical staff couldn’t be everywhere at once, and they needed help keeping on top of the material presented at key medical conferences.
•
Caravel personnel attended conference sessions and took photos of presentations and posters.
•
•
Caravel medical team wrote up summaries that covered the material presented, as well as question-and-answer sessions.
We provided daily reports to the client for storage and easy distribution using our proprietary C3 portal.

•
The client also wanted to develop a speakers bureau.
•
Caravel identified experts in the field at the national and regional levels, as well as local physician influencers. To do this, we merged top-down secondary research and bottom-up primary research with doctor and hospital claims data.
•
•
We provided in-depth profiles of relevant KOLs and intelligence information on the competitive landscape—all via our Panorama platform, which the client could view 24/7.
The client was able to use this information and Panorama’s special features to select members for and track their new speakers bureau.

A client was new to the market and lacked knowledge of their customers and key opinion leaders (KOLs) in the field.
•
•
They also wanted a more effective way to educate customers on their drug’s efficacy and safety, as well as how to identify appropriate patients.
•
Caravel proposed case-based, problem-based learning (PBL) methodology for the client’s speaker bureau program.
•
•
We developed multiple patient cases to illustrate appropriate patients for treatment, plus open-ended questions to facilitate discussion and education about the therapy.
We provided this program to the client in the form of participant and facilitator guides as program materials, in lieu of traditional presentation-based programs.

A client needed a novel program to re-engage with their key opinion leaders (KOLs) who could serve on a speaker bureau.
•
Caravel provided full support for a half-day meeting of up to 12 advisors, from recruiting the advisors themselves through providing an executive summary afterwards.
In particular, we helped:
•
•
Develop and refine content, including a slide deck that leveraged existing material to generate insights on emerging clinical data.
Manage logistics, including sourcing and securing the venue, creating invitations, and managing all on-site logistical needs.

A client’s cardiopulmonary/respiratory team needed to develop, design, and execute a half-day Regional Advisory Board.
•
•
Caravel developed a promo slide deck with the client’s branding and promotional messages. However, the FDA would need to approve this deck after granting approval for the new indication—which would take another 30 days or more. To ensure the client didn’t miss a beat, we created a second slide deck that they could use immediately upon approval of the new indication. This one was based solely on the drug’s package insert, so it didn’t require any extra FDA review.

A client was anticipating a new FDA indication for their product that would allow it to be used as a first-line therapy. They needed to prepare content that would give the clinical foundation for this approval—and they wanted it ready to use as soon as the FDA granted approval.
•
•
Caravel researched and analyzed several therapeutic areas to assess current and future unmet needs, market size per potential indication, and competition per potential indication.
•
Caravel prioritized tumor types for development efforts, based on competitive entrenchment, estimated clinical profile of company asset against current and future competitors, and access/reimbursement environment by tumor type.

A client had an asset in clinical development that showed activity against multiple tumor types. To guide investment efforts, they needed to assess and prioritize tumor types based on commercial factors.
•
•
On top of that, the client had a time problem: They’d been using an agency that didn’t work out and their first training meeting date was set—the invitations had already been sent. Now they still didn’t have updated materials, and the meeting was looming.
•
Caravel developed a single guide for participants and moderators that exceeded the client’s expectations. Client teams and 50+ faculty speakers praised the guide’s ease of use and the clinical quality of the cases.
•
Caravel’s graphics team created a new look that completely aligned with the client’s newly branded campaign.

A client needed new patient cases to illustrate recent indication updates to their label. They also wanted to consolidate new and existing case studies into a single, streamlined guide with a new, updated look.
•
Backlit and wall displays
The client was also seeking to increase interest and drive enrollment in their clinical trials.
A client’s inflammation medical affairs team wanted to introduce their business unit as an emerging leader with a robust inflammation pipeline at annual global IBD and RA conferences.
•
Caravel developed scientifically compelling IBD and RA branded materials for the conferences, including:
Brochures
Booth wall banner
Digital slide show presentations
Sponsorship items: lanyards, water bottles and cup sleeves

•
•
•
•
•
•
A global medical affairs client was stretched thin: their internal and field-based medical staff couldn’t be everywhere at once, and they needed help keeping on top of the material presented at key medical conferences.
•
Caravel personnel attended conference sessions and took photos of presentations and posters.
Caravel medical team wrote up summaries that covered the material presented, as well as question-and-answer sessions.
We provided daily reports to the client for storage and easy distribution using our proprietary C3 portal.

•
•
•
The client also wanted to develop a speakers bureau.
Caravel identified experts in the field at the national and regional levels, as well as local physician influencers. To do this, we merged top-down secondary research and bottom-up primary research with doctor and hospital claims data.
We provided in-depth profiles of relevant KOLs and intelligence information on the competitive landscape—all via our Panorama platform, which the client could view 24/7.
The client was able to use this information and Panorama’s special features to select members for and track their new speakers bureau.

A client was new to the market and lacked knowledge of their customers and key opinion leaders (KOLs) in the field.
•
•
•
•
•
They also wanted a more effective way to educate customers on their drug’s efficacy and safety, as well as how to identify appropriate patients.
Caravel proposed case-based, problem-based learning (PBL) methodology for the client’s speaker bureau program.
We developed multiple patient cases to illustrate appropriate patients for treatment, plus open-ended questions to facilitate discussion and education about the therapy.
We provided this program to the client in the form of participant and facilitator guides as program materials, in lieu of traditional presentation-based programs.

A client needed a novel program to re-engage with their key opinion leaders (KOLs) who could serve on a speaker bureau.
•
•
•
•
•
Caravel provided full support for a half-day meeting of up to 12 advisors, from recruiting the advisors themselves through providing an executive summary afterwards.
In particular, we helped:
Develop and refine content, including a slide deck that leveraged existing material to generate insights on emerging clinical data.
Manage logistics, including sourcing and securing the venue, creating invitations, and managing all on-site logistical needs.

A client’s cardiopulmonary/respiratory team needed to develop, design, and execute a half-day Regional Advisory Board.
•
•
•
Caravel developed a promo slide deck with the client’s branding and promotional messages. However, the FDA would need to approve this deck after granting approval for the new indication—which would take another 30 days or more. To ensure the client didn’t miss a beat, we created a second slide deck that they could use immediately upon approval of the new indication. This one was based solely on the drug’s package insert, so it didn’t require any extra FDA review.

A client was anticipating a new FDA indication for their product that would allow it to be used as a first-line therapy. They needed to prepare content that would give the clinical foundation for this approval—and they wanted it ready to use as soon as the FDA granted approval.
•
•
Caravel researched and analyzed several therapeutic areas to assess current and future unmet needs, market size per potential indication, and competition per potential indication.
Caravel prioritized tumor types for development efforts, based on competitive entrenchment, estimated clinical profile of company asset against current and future competitors, and access/reimbursement environment by tumor type.

A client had an asset in clinical development that showed activity against multiple tumor types. To guide investment efforts, they needed to assess and prioritize tumor types based on commercial factors.
•
•
•
On top of that, the client had a time problem: They’d been using an agency that didn’t work out and their first training meeting date was set—the invitations had already been sent. Now they still didn’t have updated materials, and the meeting was looming.
Caravel developed a single guide for participants and moderators that exceeded the client’s expectations. Client teams and 50+ faculty speakers praised the guide’s ease of use and the clinical quality of the cases.
Caravel’s graphics team created a new look that completely aligned with the client’s newly branded campaign.

A client needed new patient cases to illustrate recent indication updates to their label. They also wanted to consolidate new and existing case studies into a single, streamlined guide with a new, updated look.
•
•
•
•
Where We Work
We are headquartered in the Bay Area with full-time staff and
offices in New Jersey and Atlanta
Caravel Group Communications offers specialty consulting and marketing services for the life sciences industry.
